
Varithena – Endovenous Chemical Ablation of Varicose Veins
Varithena (Polidocanol Foam 1%) is an FDA-approved treatment indicated for the treatment of varicose veins of the legs. It is indicated to remedy saphenous vein insufficiency of the legs which causes varicose veins to develop.
The treatment can therefore often cure varicose veins and prevent them from recurring on the legs. It can be used to treat varicose veins in the upper and lower legs. The therapy can improve the appearance of visible varicose veins and also improve or resolve the symptoms related to vein disease in the legs. It has been approved for the treatment of incompetent/abnormal leg veins that have developed vein valve failure, leading to bulging painful, and unsightly blue veins on the legs. These abnormal veins are referred to as saphenous veins or varicose vein branch tributaries. The underlying condition that leads to the defective veins is referred to as venous insufficiency. The medication has been tested and approved for the treatment of the great saphenous vein and the accessory saphenous veins of the legs as well as the branch varicosities emanating from these abnormal veins. It is indicated for small, medium, and large diameter veins, even veins >12 mm in diameter. It is effective in the treatment of tortuous and recurrent varicose veins as well. the medication is administered intravenously (through an IV) by a physician with the use of radiologic imaging. This Varithena procedure also is sometimes referred to as “endovenous chemical ablation” or “ultrasound-guided sclerotherapy.” It is considered safe and effective.
What is Varithena Foam?
Varithena foam is a cohesive low-nitrogen microfoam that has been demonstrated to have consistent performance in the treatment of leg vein disease. It includes a mixture of a liquid polidocanol medication and natural gases which are micro-bubbles of predominantly oxygen and carbon dioxide. The added gases help to improve the effectiveness of the drug in the resolution of abnormal veins. It is designed to fill an abnormal vein lumen by displacing the blood present. The drug contact then causes a chemical reaction at the level of the internal vein wall (endothelium) that destroys the vein efficiently, causing the vein to absorb into the body. It was studied in 12 clinical trials of over a thousand patients and showed good effectiveness and a good safety profile.
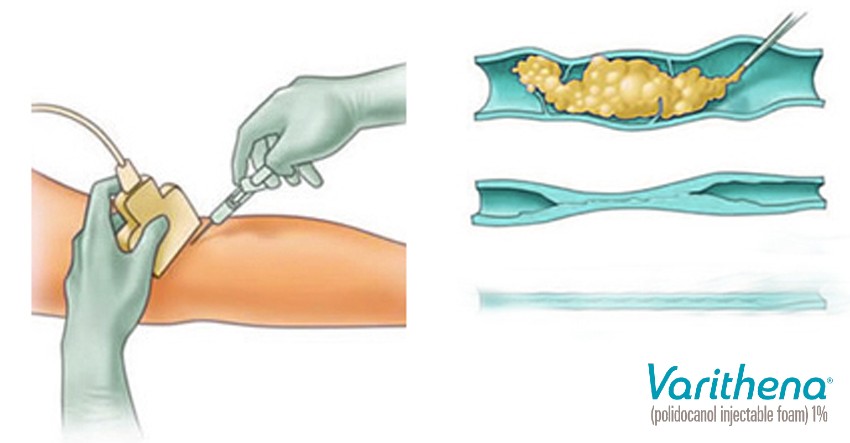
Is Varithena the Same as Foam Sclerotherapy?
Yes, it is essentially the same type of treatment but utilizes a unique patented form of sclerotherapy medication. Other terms that are often used to describe this Varithena treatment are “foam sclerotherapy,” “endovenous chemical ablation,” or “ultrasound-guided sclerotherapy”. The “foam” refers to the appearance of the medication, which looks like foam shaving cream.
This type of treatment has been performed by vein physicians for many decades with good results. But there was recently additional research and testing of this method in the United States which lead to the FDA approval for this particular form of polidocanol foam, marketed through Biocompatibles, Inc. What is unique to Varithena is the proprietary canister device that is used to create and deliver the foam medication. This device produces a cohesive low-nitrogen micro-foam with controlled density and bubble size, with unique oxygen to carbon dioxide gas mixture of 65% to 35%. This foam mixture helps to maximize the contact time between the vein wall and polidocanol chemical, therefore theoretically maximizing the effectiveness of the drug. Foam sclerotherapy can also be utilized with other types of sclerotherapy medications as well.
What is the difference?
The main difference between Varithena and typical foam sclerotherapy is that Varithena utilizes a unique patented micro-foam medication and it is often performed for larger or deeper veins compared to standard sclerotherapy. The other difference is that ultrasound is always utilized when administering this medication.
What type of imaging is utilized during this type of sclerotherapy?
Ultrasound. Unlike traditional sclerotherapy, foam sclerotherapy is performed utilizing ultrasound imaging. Foam medications have the unique property of being able to be directly visualized easily with an ultrasound machine. So in order to assure precise and accurate injection of the medication locally into the abnormal vein, an ultrasound machine applied to the skin surface is used to visualize the vein during the injection. Only a small amount of polidocanol chemical is injected into the lumen of the abnormal vein until it fills the vessel and then the injection is discontinued.
Is this type of therapy covered by health insurance?
Varithena ultrasound-guided sclerotherapy is often covered by insurance for the treatment of symptomatic varicose veins of the legs. Whether you will be a good candidate for the therapy will be determined by your doctor.
For further information about this form of vein treatment, please contact our vein center at (512) 220-5401 or use the “Contact Us” form on this website.


